
How the pH of cosmetic products affects your skin health

Have you ever wondered what the pH was of the facial cream you use every day? What about the pH of your favorite foundation or primer? For many people, this thought does not cross their mind. Most people only look at how a product performs at a more superficial level such as how it makes their skin look upon application. However, unbeknownst to many, a product at the wrong pH can be detrimental to your skin. Perhaps at this moment, you are thinking, “Ok. But what on earth does pH even mean?”
Simply stated, the pH is a measure of an aqueous substance’s acidity or basicity/alkalinity. The so-called acid mantle of the skin consists of compounds that come from both the external environment and from within the body. These include metabolic by-products of bacteria, chemicals produced within the body sent to the surface of the skin such as lactic acid and butyric acid (both of which are contained in sweat), acidic lipids, and fatty acids from the sebaceous glands. It is believed the lactic acid from sweat sinks back into the skin and in doing so acidifies the uppermost layers of the stratum corneum (SC). Additionally, there are metabolic products such as uronic acid and pyrrolidone acid and H+ (hydrogen ions) created by cellular transporters like the sodium-hydrogen exchanger. All of these contribute to the acidic nature of the outermost layer of our skin’s SC.
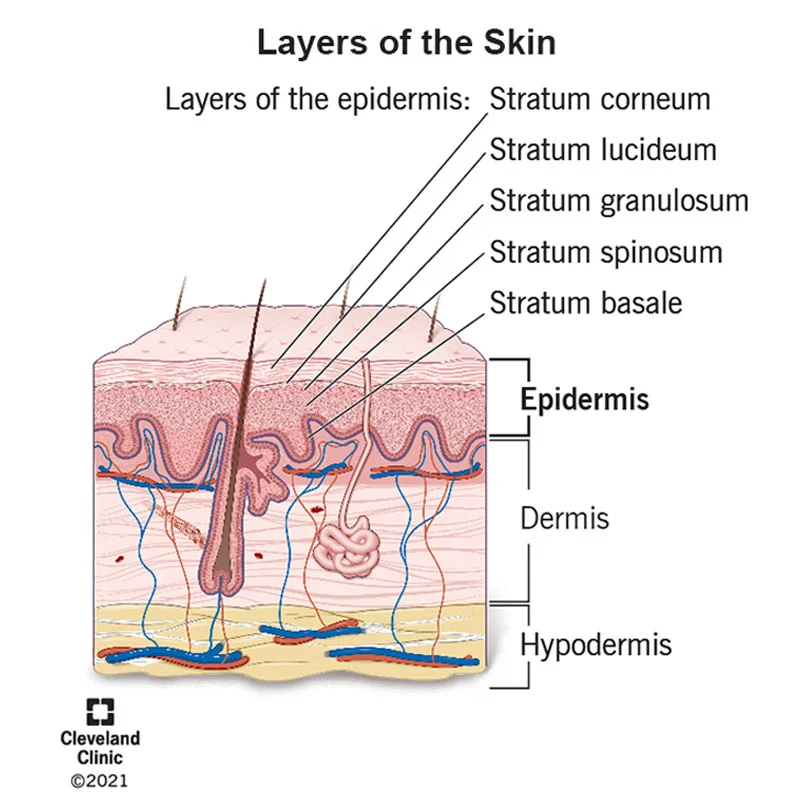
You may now be wondering what the SC is. The SC is the top layer of the epidermis also referred to as the skin barrier. The SC keeps out unwanted material (such as allergens and irritants) and keeps water from escaping the body. The acidic nature of the skin barrier is necessary for homeostasis because the ceramide producing proteins in the skin need an acidic environment to function. Also, the low pH promotes skin barrier synthesis. Therefore, a stronger skin barrier is created in an acidic environment.
One study done by Ohman and Vahlquist (1998) determined that skin surface pH was about 4.5 while another study measured the pH as 4.7. Both figures are significantly lower than the pH of 5.5 traditionally used for formulation. These researchers proceeded to do tape stripping on subjects to remove the upper layer of the skin on the forearm. As they got progressively deeper into the skin, the pH rose from 4.5 to 7.0. They concluded there was a relationship between the pH level and the level of the previously described chemical species, the amount of which changed with the depth in the epidermis. This supports the idea that acidification not only occurs from the inside out, but also from the outside to the in, for example, by the movement of lactic acid to the inside. So, what happens when the acidity of the skin barrier is altered?
If the pH of the SC is increased, for example by putting more basic/alkaline substances on the skin, the activity levels of serine proteases (proteins that degrade protein) increase and the structures necessary for SC cohesion (the corneodesmosomes) degrade at a faster rate which negatively impacts SC integrity.
Skin at a low pH (under 5.0) has a better barrier and is more hydrated, Acidic skin surface pH is also less likely to get dermatitis and it displays significantly less scaling as compared to skin with a pH over 5.0. A skin surface with a low pH is also more resistant to colonization by pathogenic microbes, which is done by maintaining growth of the resident, natural, flora on the skin and inhibiting the growth of pathogenic, transient flora. If the pH is increased, this state of being is reversed.
Since research has shown that a skin surface pH below 5.0 is necessary for optimal skin health and function, you might think that most formulations for the skin would have a pH below 5.0. But this is not the case. Only a small fraction of the formulations analyzed has a preferred pH value of around 4.5.
In conclusion, knowing the pH of the products you put on your body, especially your face, is important. Products with pHs below 5.0, more specifically around 4.5 or 4.7, will maintain your skin’s natural flora and the structural integrity of your skin barrier. Moisture loss will also be reduced. In this environment, the natural processes occurring on the outermost regions of the epidermis will be maintained. On the other hand, products with a pH above 5.0 can disrupt the growth of natural flora on your face and encourage the growth of pathogenic microorganisms. Additionally, the structure of the skin barrier can become compromised. This will result in moisture loss, higher susceptibility to skin conditions such as dermatitis, and increased skin scaling.





